Because many treatments’ effects vary depending on the individual, researchers argue that effectiveness should be evaluated per patient. Yet the simple evaluation method that’s been tested and refined for 30 years, called an n-of-1 trial, is rarely used. Why?
Most top selling US drugs for chronic conditions offer above average relief for only a fraction of treated patients. So, for nearly 30 years, researchers have advocated single patient treatment experiments, also known as an n-of-1 trials.
An n-of-1 trial uses standard scientific methodology—including placebos, blinding, random block rotation, crossovers, washouts and statistical analysis—to determine a drug’s efficacy for an individual patient. Their rigor and specificity mean that n-of-1 trials “facilitate finely graded individualized care, enhance therapeutic precision, improve patient outcomes, and reduce costs,” according to a 2013 review of 2,154 single patient trials.
N-of-1 trials counter cognitive biases—confirmation bias, a desire to please the doctor, the placebo effect, the natural progression of an illness, to name a few—that can commonly skew the unstructured drug sampling, aka trial and error method, that most clinicians rely on.
To date, though, we’ve seen a failure of all attempts to provide off-the-shelf n-of-1 services that the average doctor might use for a patient.
“We spend so much effort on precision in diagnosis, yet we have very little precision about treatment,” says Eric Larson, Executive Director and Senior Investigator, Kaiser Permanente Washington Health Research Institute.
Why? The excellent 2008 paper What Ever Happened to N-of-1 Trials? Insiders’ Perspectives and a Look to the Future summarizes explanations.
1) The average physician’s toolbox doesn’t include the necessary statistical hammers and nails to do a meaningful analysis. “In clinical practice, physicians use evidence and experience to generate a list of treatment options. Patients and physicians move down the list based on trial and error.”
2) N-of-1 trials require physicians to squeeze another process and script into their already-packed days. “Clinicians must explain the idea to their patients, see them regularly throughout the trial, and evaluate the results jointly at the trial’s end. … Taking the time to sell an unfamiliar concept—let alone follow through on the logistics—might be untenable.”
(A 2017 history of n-of-1 trials offers a more prosaic take: “‘did the treatment help?’ is too easy, and has too much face validity, compared to the more onerous substitution of a formal N of 1 trial.”)
3) Beyond the pragmatic challenges, N-of-1 trials force physicians out of their “cure-dispenser” role, the study noted. “While clinicians understand that the practice of medicine involves uncertainty, they are not necessarily comfortable with it. Recommending that a patient enroll in an n-of-1 trial acknowledges this uncertainty, suggesting that the physician does not really know what is best. It is much easier—and arouses far less cognitive dissonance—to simply write a prescription.”
As Gordon Guyatt, who pioneered research at McMaster University demonstrating the empirical superiority of N-of-1 trails, explains resistance to the n-of-1 innovation: “[Physicians] tend to have ways that they’ve learned to operate where they’re comfortable, and it’s hard to move outside these ways. It’s hard to do something extra that’s going to take time, and energy, and initiative.”
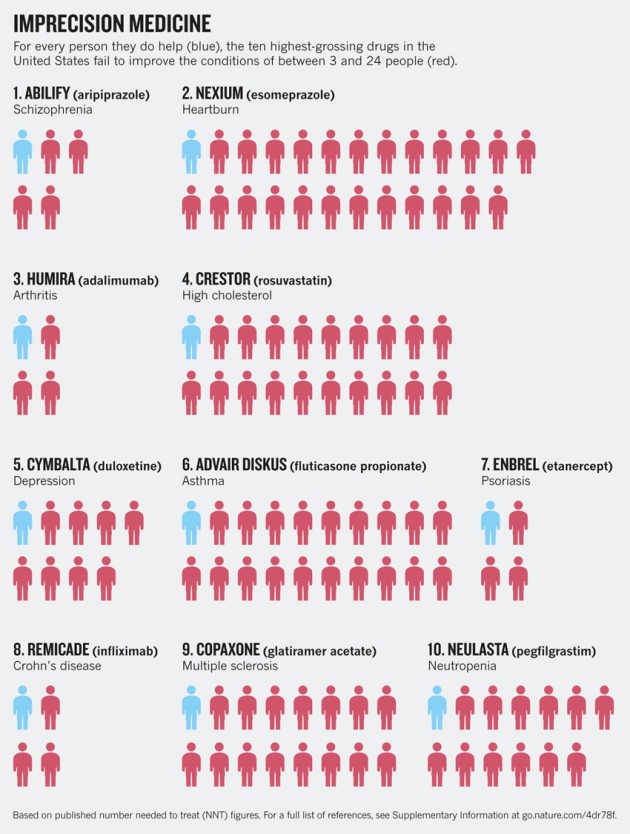
Some argue N-of-1 trials are long overdue. “Every day, millions of people are taking medications that will not help them,” argues Nicholas J. Schork, director of human biology at the J. Craig Venter Institute in La Jolla, California, USA. “The top ten highest-grossing drugs in the United States help between 1 in 25 and 1 in 4 of the people who take them (see chart below.) For some drugs, such as statins — routinely used to lower cholesterol — as few as 1 in 50 may benefit. There are even drugs that are harmful to certain ethnic groups because of the bias towards white Western participants in classical clinical trials.” Schork believes the time is ripe for n-of-1 trials. “Physicians are having to become more acutely aware of the unique circumstance of each patient — something most people have long called for.”
To learn more about creating your own n-of-1 test for your own symptoms, engage with the GuideBot below!